The natural oils and fatty acids
Natural oils are found in both animals and vegetables and are separated from the feedstock by mechanical means, by presses, or by solvent extraction (using a solvent to dissolve the oil before separating the solvent from the remaining solid matter ). Depending on their melting point they are commonly classified as 'lards' (those of animal origin that are semi solids at room temperature) or simply 'oils', of both animal and vegetable origin, when they are liquids. Examples of animal (vertebrate) oils are those derived from cattle or fish, such as tallow, lard, sardine etc . There are significantly more varieties amongst the vegetable oils and the oils may be derived from the flowers, sap, seeds, or even the entire plant. Examples of vegetable oils are canola, castor, corn, linseed, olive, soybean, tung etc.
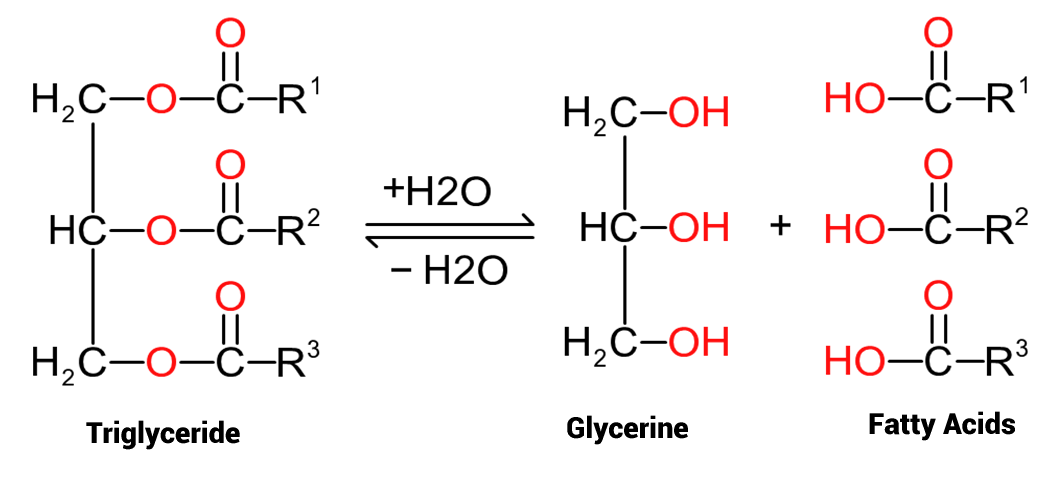
As readily noted, there are large differences between the appearance and use of the various oils, and this boils down to their composition. These oils are composed of molecules based on one glycerol (glycerine) molecule together with three fatty acid molecules, as per figure 1, and are hence known as 'triglycerides'. The glycerine molecule is common to all triglycerides, but the composition of the fatty acids varies depending on the specific oil - which, therefore, accounts for the differences in the oils derived from different sources. Referring to figure one we have a triglyceride on the left side and the 'hydrolysed' components, glycerine and fatty acid, on the right. As each fatty acid on a triglyceride amy be different, they have been annotated as 'R1', 'R2' and 'R3' as a crude method of differentiation of the fatty acids that compose different oils. In between the two sets of molecules is a 'bidirectional arrow' which indicates a reaction, and the reaction can go 'both ways'. In the first instance, from left to right, a triglyceride can be broken down by reaction with water (and normally heating plus a catalyst) to form separate glycerine and fatty acid molecules. In the reverse direction, from right to left, fatty acids and glycerine can be reacted, with dehydration (loss of water), to form a triglyceride and water via a condensation reaction. The first reaction, the breakdown of triglycerides to form glycerin and the relevant fatty acids is an industrially significant one, not only is it possible, by this route to generate fatty acids, but also the commercially significant glycerin (glycerol), with its multiple applications across many industries.
Turning to the fatty acids, these are numerous in kind and complexity. At one end one has a carboxyl group (acid group) and a 'fatty acid chain'. The fatty acid chain is composed of numerous carbon atoms, all linked together in a chain. The combination of a carbon chain and a carboxylic group gives rise to the chemical name for these molecules as 'carboxylic acids'. The number of carbon atoms differs from one fatty acid type to another and dictates their nomenclature. For instance one might have a carboxylic acid with 5 carbon atoms (pentanoic acid), 6 carbon atoms (hexanoic acid), 8 carbon atoms (Octanoic Acid) etc. However, the fatty acids that make up natural oils are far more complex in nature than these simple types and might have up to 24 carbon atoms in their chains.
The linkage between the carbon atoms is also important. The majority of the bonds between carbon atoms are simple 'single bonds' but there is a second bond that also occurs, known as a 'double bond', alternatively called 'unsaturated bonds'. Fatty acids that are composed solely of carbon-carbon single bonds are termed 'saturated fatty acids', whilst those that contain one, or more, unsaturated (double) bonds are termed 'unsaturated fatty acids' (Those termed 'polyunsaturates' have two or more double bonds). Both of these terms will be known to many people, but more so those who are interested the content of Omega 3, Omega 6 and Omega 9, the 'essential oils',as found in a particular food or health product.
| Saturated Fatty Acids | Unsaturated Fatty Acids | Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid | Lauric | Myristic | Palmitic | Stearic | Oleic | Ricinoleic | Linoleic | Rumenic | Linolenic | Eleostearic | Saturates | Unsaturates | Iodine Value | Solid/Liquid |
| Carbon Atoms | 12 | 14 | 16 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | ||||
| Double Bonds | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 3 | 3 | ||||
| Hydroxyls (OH) | 1 | |||||||||||||
| Beef Tallow | 3 | 24 | 19 | 43 | 3 | 1 | 46 | 47 | 50 | Semi Solid | ||||
| Castor | 1 | 1 | 1 | 3 | 89 | 4 | 3 | 96 | 86 | Liquid | ||||
| Dehydrated Castor | 1 | 1 | 1 | 3 | 4 - 27 | 60 - 90 | 4 | 96 | 123 | Liquid | ||||
| Coconut | 47 | 18 | 9 | 3 | 6 | 2 | 91 | 9 | 10 | Liquid | ||||
| Corn | 3 | 12 | 2 | 27 | 59 | 1 | 14 | 87 | 123 | Liquid | ||||
| Linseed | 3 | 6 | 4 | 19 | 15 | 57 | 10 | 91 | 180 | Liquid | ||||
| Olive | 9 | 3 | 80 | 6 | 1 | 10 | 89 | 84 | Liquid | |||||
| Palm | 1 | 43 | 5 | 39 | 11 | 49 | 50 | 53 | Liquid | |||||
| Sardine | 6 | 10 | 2 | 24 | 59 | 1 | 18 | 82 | 182 | Liquid | ||||
| Soybean | 3 | 11 | 4 | 24 | 54 | 7 | 15 | 85 | 130 | Liquid | ||||
| Sunflower | 3 | 5 | 4 | 24 | 61 | 1 | 10 | 89 | 131 | Liquid | ||||
| Tall | 3 | 4 | 59 | 36 | 3 | 96 | 132 | Liquid | ||||||
| Tung | 3 | 5 | 4 | 9 | 82 | 5 | 95 | Liquid | ||||||
Table 1 above lists a selected number of oils and their approximate fatty acid compositions. Note that the actual fatty acid composition is affected by a number factors, the variety of the plant and it's growing conditions. For example, oil seeds harvested at one time of the year might have different oil compositions if harvested at a different time of the year, oils derived from trees, e.g. Tall Oil from Pine trees, have different oil compositions depending on whether the pine trees are grown in temperate or cold climates.
It should also be noted that not all of the fatty acids in a particular oil have been listed. There are other fatty acids, that occur in small quantities, that have been omitted to reduce the table size, hence the totals of the saturated and unsaturated fatty acids might not equal 100%.
| Saturated Fatty Acids | ||
|---|---|---|
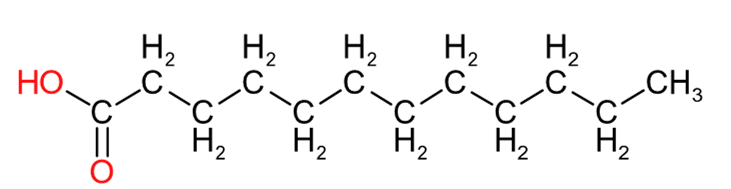
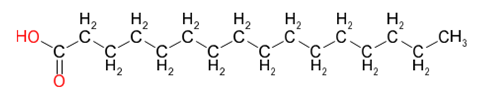
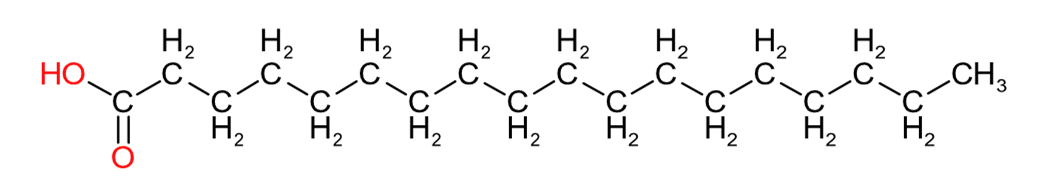
| Lauric Acid (C12:0) | Palmitic Acid (C16:0) | Stearic Acid (C18:0) |
 |
 |
 |
| Unsaturated Fatty Acids | ||
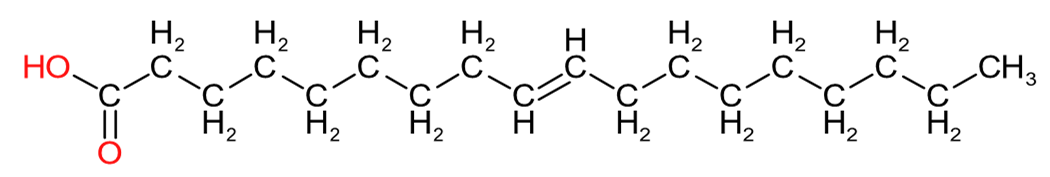
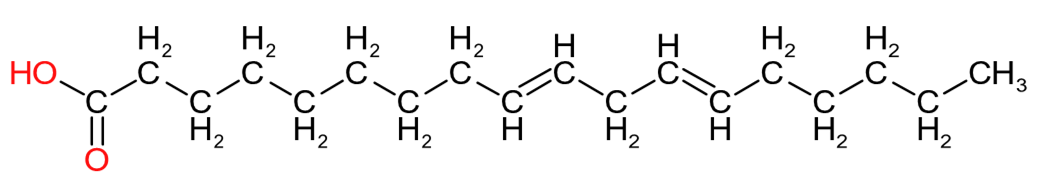
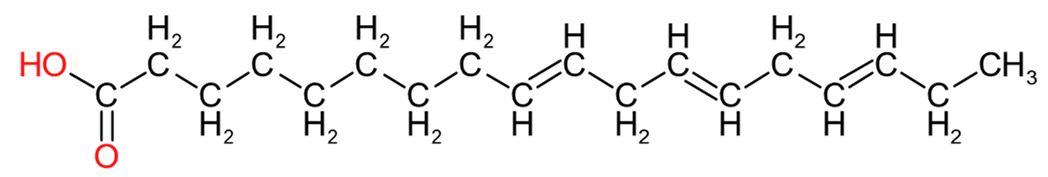
| Oleic Acid (C18:1) | Linoleic Acid (C18:2) | Linolenic Acid (C18:3) |
 |
 |
 |
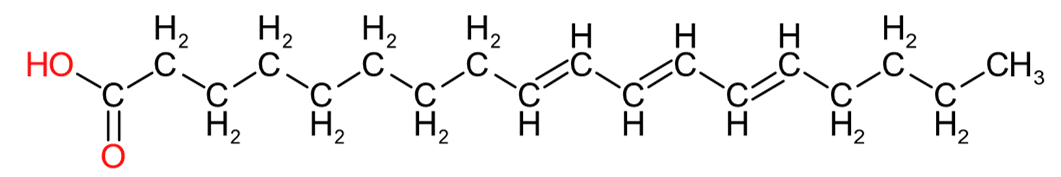
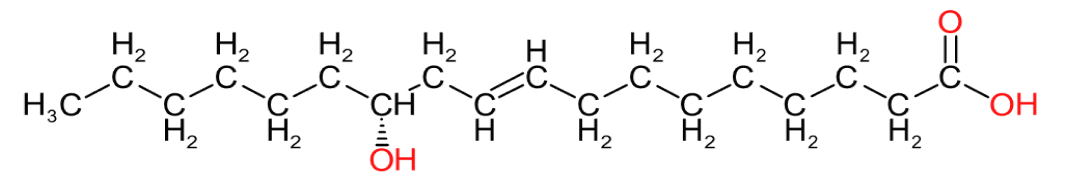
| Eleostearic Acid (C18:3) | Ricinoleic Acid (C18:1) | Rumenic Acid (C18:2) |
 |
 |
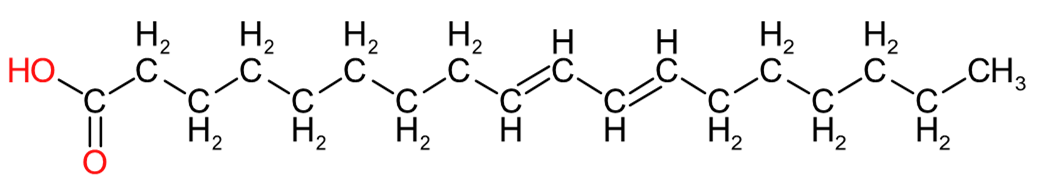 |
Table 2 provides an insight into the structures of some of the common fatty acids that are found in natural oils, where C = carbon, H= hydrogen, and O = oxygen. The molecules have been drawn in a linear fashion to facilitate their incorporation in the table. The actual shape of the molecules, are more 3 dimensional in nature and, rather than linear carbon chains, they are bent at various angles with some molecules being curved and/or twisted in nature. Additional information will be provided in the 'Chemistry' sections on the relevant resin pages.
In reality, the fatty acid structures look more like that of the Figure 2 (Linoleic acid). To conserve space, the fatty acid molecules in the tables have been drawn in a linear fashion.
The carbon-carbon bond angle remains at 109.5° but the presence of carbon:carbon double bonds give rise to isomerism as the carbons on either side of the double bond are inhibited from rotation.