Esterification Reactions
The Esterification Process
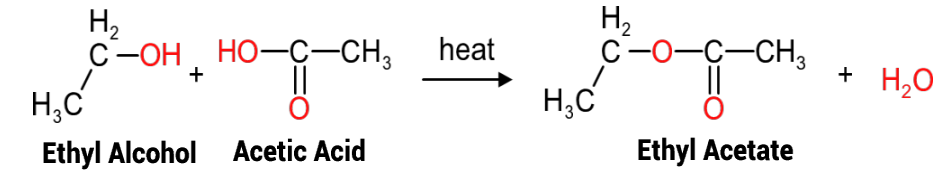
Esterification reactions are the primary reactions used in the production of polyester resins, such as the alkyds, modified alkyds, saturated and unsaturated polyesters etc. The reaction is akin to inorganic acid/base reactions but rather than forming a salt plus water, the reaction is between organic molecules, for example between the (acidic) carboxyl group of acetic acid, and the (basic) hydroxy group of ethanol (Figure 1), producing an ester linkage. and water
These reactions are, in reality, reversible and the equation should rather be written as per Figure 2. The 'bidirectional' (double) arrow indicates that the reaction can proceed in either direction. From left to right, the loss of water will result in the formation of ethyl acetate, whilst from right to left, the addition of water will produce ethanol and acetic acid.
In practice one ends up with a fixed proportion of reactants and products in equilibrium, depending on the process conditions used.
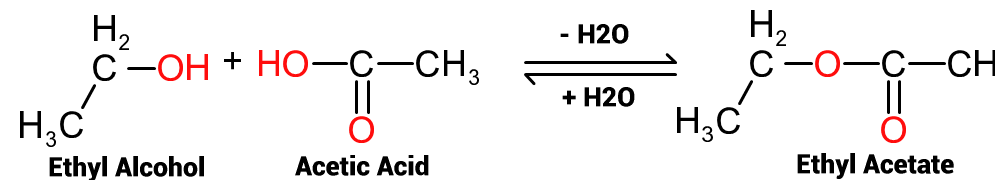
Le Chatelier's Principle states that, in a situation of dynamic equilibrium, a change to the concentration of one or other components will cause a change in the equilibrium concentrations. Which practically means that if one adds more ethyl alcohol to the mixture, it will force the equilibrium to the right (more ethyl acetate will be produced). Similarly, reducing the water of reaction concentration in the mixture will increase the proportion of ethyl acetate produced. This later approach is the one that is undertaken as it is easy to distil off the water of reaction from the reaction mixture, both in the laboratory and in production, to increase the quantity of ethyl acetate produced.
In certain circumstances, acid groups from the same molecules can react, via dehydration (loss of water) to form 'anhydride groups' as indicate in Figure 3, where two molecules of acetic acid will react to form Acetic anhydride with the loss of one molecule of water.
Note that the reaction is bidirectional and the addition of water will reverse the reaction
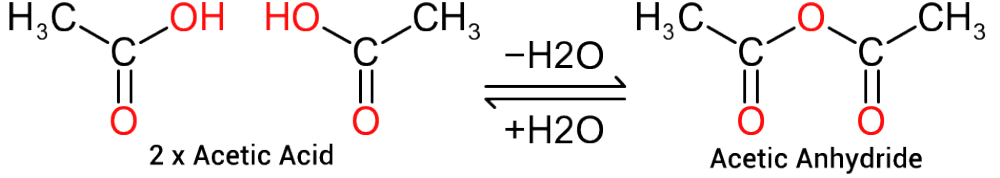
Anhydrides are very convenient for the manufacture of esters as they readily react with alcohols at very low temperatures, with some anhydride/alcohol reactions being quite exothermic.
The grouping O=C-O-C=O is generally referred to as the 'anhydride ring' and the reaction that breaks this ring (such as reacting with water) as 'ring opening.'
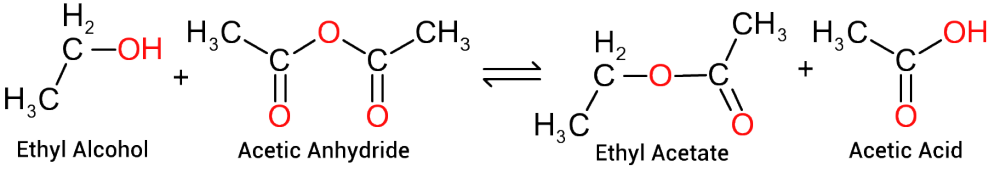
The reaction between ethanol and acetic acid produces molecules of ethyl acetate and that is where the reaction ends. To produce a polymer we require reactants that have a higher degree of functionality (e.g. molecules containing more than one basic group and more than one acid group per molecule) If we were to react equimolar quantities of monoethylene glycol (2 hydroxyl groups) and adipic acid (2 carboxyl groups), we would initially produce a molecule of ethylene adipate, as depicted in Figure 5, which contains both an hydroxy group at one end and a carboxylic acid group at the other.
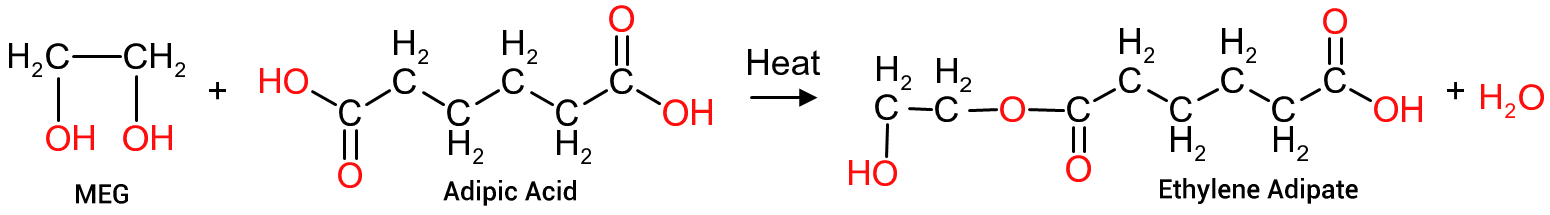
If we continue the reaction we can join 2 molecules of ethylene adipate to form a second molecule, of twice the size, with a further loss of a molecule of water, as depicted in Figure 6. As this new molecule has at least 2 identical, repeating molecules, it can now be termed a polymer.
The new molecule again has both a hydroxy and a carboxylic group so these molecules can again be reacted to form a molecule 4 times the size of the original molecule of ethylene adipate. In theory one can continue the reaction until one has a gigantic polymeric molecule (that would be insoluble in most common solvents), terminated at one end with an hydroxy group and the other end with carboxylic acid group .
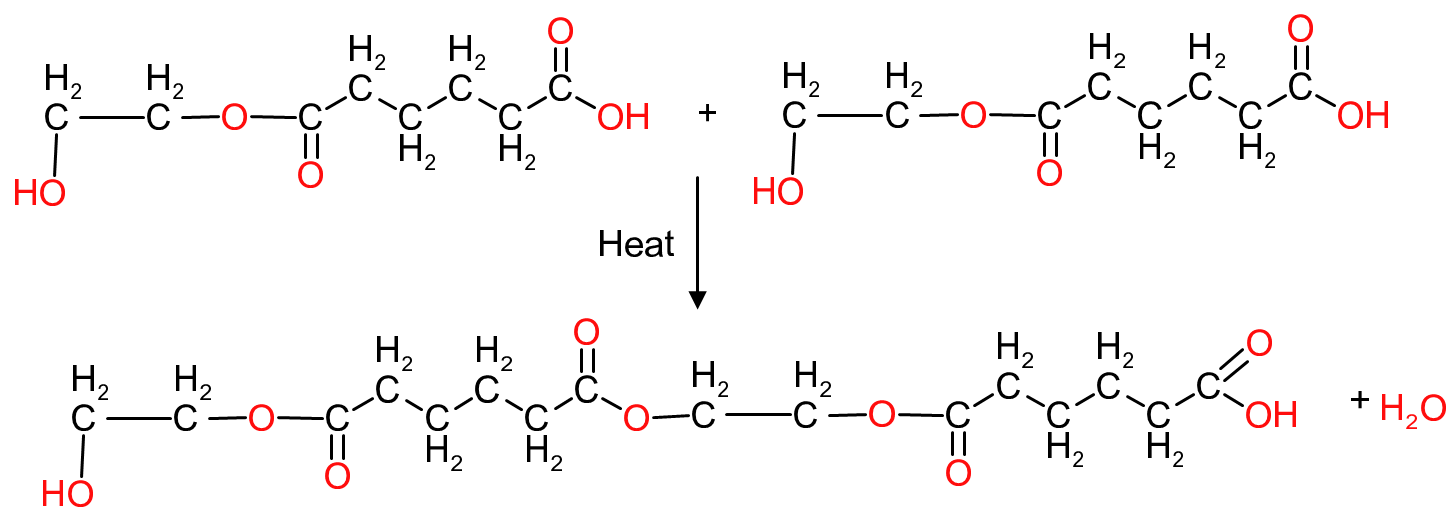
To prevent the polymer growing to unacceptable proportions, such as when equimolar concentrations are used, it is necessary to increase the quantity of either the basic or acidic molecules such that the polymer ends are terminated either by an acidic or basic group.
The most common approach is to add the hydroxy molecule in excess. If we were to react 3 molecules of ethylene glycol with 2 moles of adipic acid, the final polymer would be as depicted in Figure 7, with the polymer now containing hydroxyl groups at each end thus preventing any further reaction.
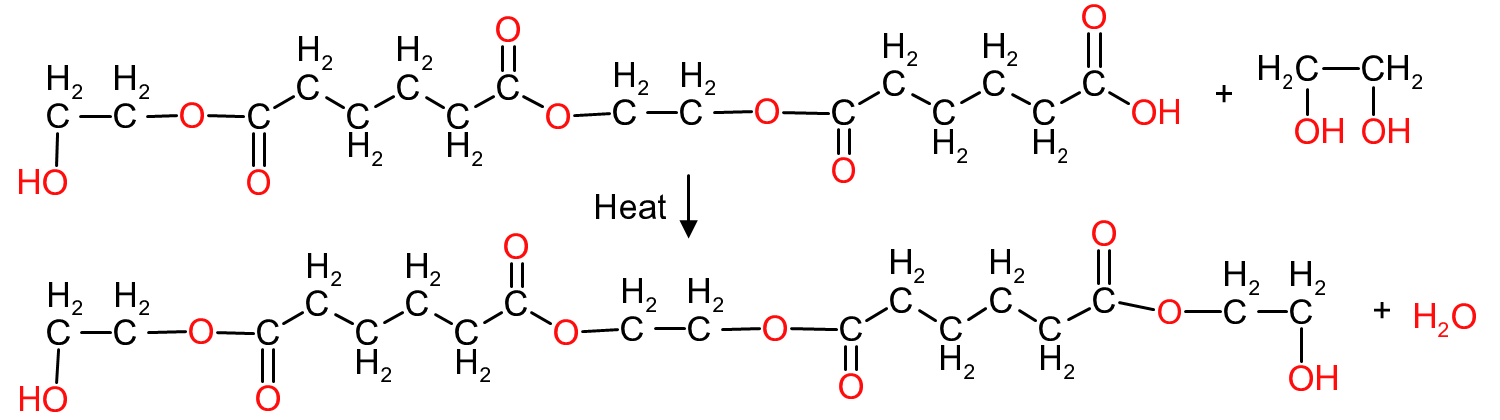
The molecular mass of the polymer in Figure 7 would be 3 times the molecular mass of ethylene glycol plus 2 times the molecular mass of adipic acid minus 4 times the molecular mass of the water lost during ester group formation during the esterification: (3*62.1) + (2*146) - (4*18) = 406.3 AMU (Atomic Mass Units)
In practical terms, a polymer of this molecular mass would be very soft, have low durability and a very low viscosity when dissolved in a suitable solvent. Practically, the majority of polymers would have molecular masses in excess of 1,000 AMU.
To increase the molecular mass one would simply adjust the ratio between the glycol and the acid. For instance, the current ratio is 3:2 (1.5:1) ethylene glycol to adipic acid and if we lowered the ratio to say 8:7 (1.14:1) ethylene glycol to adipic acid the polymer molecular mass would become:
8 molecules of ethylene glycol + 7 molecules of adipic acid - 14 molecules of water of reaction = (8*62.1) + (7*146) - (14*18) = 1,266.8 AMU
Azeotropic Distillation
Azeotropic distillation is similar to normal distillation in that the goal is to remove the water of reaction during processing. However, with azeotropic distillation a solvent is added to the reaction mix to increase the efficiency of water removal. These solvents effectively use a process where the solvent actively aids the extraction of the water from the reaction mix by forming an azeotrope.
An azeotropic mixture is one where the combined solvent plus water combination has a lower boiling point than either the solvent, or water has, on their own. There are a number of solvents that are used to form an azeotrope with water as outlined in Table 1 below. Please note that this table is just an example of a few azeotropes. The only one that is commonly used in the resins covered here, is xylene, because it demonstrates low water solubility after condensing (such that one is only recycling a very small percentage of water back into the reaction mix) and the water layer has very little xylene concentration (making it easier to treat for environmental reasons). The other potential candidate for azeotropic distillation would be toluene. However the later is extremely prone to static build-up and presents a high risk of fire unless properly handled.
| Solvent | Boiling Point | Azeotrope Temperature | Azeotrope Phase | Solubility of solvent in Water | Solubility of water in Solvent | |
|---|---|---|---|---|---|---|
| °C | °C | Solvent (%) | Water (%) | % at ±25 °C | % at ±25 °C | |
| n-Butanol | 118 | 93 | 64 | 36 | 6.5 | 22.4 |
| Butyl Acetate | 126 | 90 | 71 | 29 | 0.7 | 1.6 |
| Toluene | 111 | 84 | 82 | 18 | 0.05 | 0.03 |
| Xylene | 139 | 92 | 68 | 32 | 0.02 | 0.01 |
In an azeotropic distillation, around 1 to 3% of solvent is added to the reaction mixture based on the total mass of the reactants. On heating, when the mixture attains the azeotropic temperature of the specific solvent/water combination, it begins to boil. The vapours produced contain both water and solvent, in the percentages indicated in the azeotrope phase, which would be, for example using xylene, 68% xylene and 32% water. The boiling point of the mixture would be 93°C (i.e. 7°C below that of water itself and 47°C below that of xylene), if we were only considering a mixture of xylene and water. However, the presence of a solute in the reaction mixture (which in our case would be the polymer reactants and any polymer produced thus far) will increase the observed boiling point by a considerable amount. This phenomenon is termed: "boiling point elevation".
The vapour emanating from the reaction mixture cooled quickly to a low temperature, via a condenser and collected in an external tank, commonly referred to as a separator tank. On cooling, the vapour phase separates into 2 liquid layers, the lower layer, water, contains only 0.01% xylene (at ±25°C), whilst, conversely, the xylene layer only contains 0.01% of water. The xylene layer from the separator tank is continuously recycled back into the reaction mix until the reaction is complete, at which point the remaining xylene is distilled off from the finished product, or left in situ if the final product uses xylene as one of its solvents.
It must be remembered, particularly in the case of polymer production, as the molecular mass of the polymer increases, so too will the boiling point of any water, solvent and azeotrope in the mixture. As such, the temperature of the reaction is normally increased, as the reaction proceeds, to ensure that the mixture continues to boil, and the azeotrope evaporate, until the termination point of the reaction is reached. In some systems vacuum may be applied to reduce the boiling point temperature such that it does not become excessive.
Interestingly, the measure of the boiling point of a given quantity of polymer dissolved in a specified amount of solvent was used as an indication of the molecular mass of the polymer. The higher the boiling point, the higher the molecular mass of the polymer.
Problem areas associated with esterification reactions
In the real world there are always complications that arise in formulating resins that are polymerised via esterification reactions. Some of the areas to watch out for are as follows:
- Volatile raw materials: A number of the glycols used in formulating saturated and unsaturated polyesters have boiling points close to, or below, the temperatures used in the polymerisation process. For instance, mono propylene glycol (B.Pt 188°C) and mono ethylene glycol (B.Pt 197°C) both have boiling points below normal processing temperatures (≥210°C). As such, an additional amount, over the desired quantity, needs to be added to compensate for loses during processing. Unfortunately the compensation that is required varies with both the processing conditions and the design of the actual equipment used for processing (i.e. reactors that are not identical will require different amounts of glycol compensation).
- Side reactions:
Unwanted side reactions, can occur during processing. For example, at temperatures above 210°C the hydroxyl groups on 2 mono ethylene glycol molecules can react, especially under the acidic conditions these reactions are normally performed under, to form one molecule each of diethylene glycol and water, thereby reducing the hydroxyl functionality from 4 to 2. I.e:
HO-(CH₂)₂-OH + HO-(CH₂)₂-OH → HO-(CH₂)₂-O-(CH₂)₂-OH + H₂O - Oxidation: The presence of oxygen, or nitrous oxides, during processing can adversely affect the colour and/or viscosity of the final product. In order to exclude both these gases from the reaction vessel, it is normal to use an inert gas to blanket the vessel contents during processing. Nitrogen is the most commonly used gas for this purpose but carbon dioxide may also be used.
- Processing conditions: Particularly with the alkyd resins, variations in the processing conditions can adversely affect the quality of the finished product. Varying the process temperature and/or the processing time can result in variations of the final product properties.