Alcoholysis of Oils
Overview
Natural oils are found in both animals and vegetables and are separated from the feedstock by mechanical means, by presses, or by solvent extraction (using a solvent to dissolve the oil before separating the solvent from the remaining solid matter ).
Depending on their melting point they are commonly classified as 'lards' (those of animal origin that are semi solids at room temperature) or simply 'oils', of both animal and vegetable origin, when they are liquids.
Examples of animal (vertebrate) oils are those derived from cattle or fish, such as tallow, lard, sardine etc.
There are significantly more varieties amongst the vegetable oils and the oils may be derived from the flowers, sap, seeds, or even the entire plant. Examples of vegetable oils are canola, castor, corn, linseed, olive, soybean, tung etc.
The interrelationship between a triglyceride and the associated fatty acids (marked R¹, R² and R³) is indicated in Figure 1, to the right. Heating a triglyceride in the presence of water and a catalyst will hydrolyse the oil into its component parts, glycerine and associated fatty acids. Conversely, reacting 3 molecules of fatty acids with 1 molecule of glycerine, and removing the water of reaction, will revert to the original triglyceride.
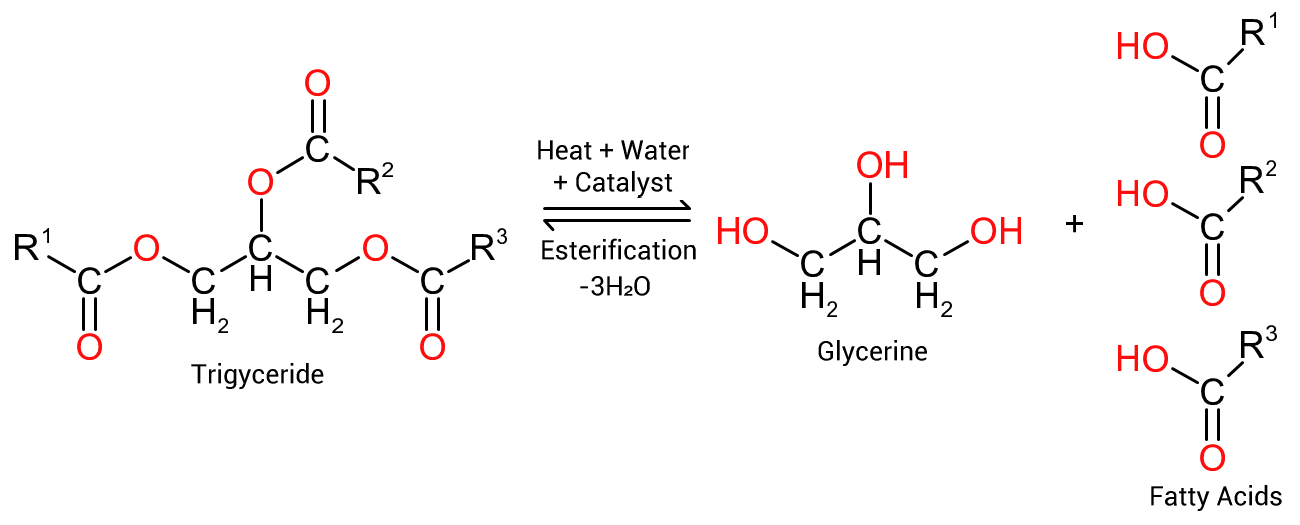 Figure 1: Hydrolysis of triglycerides into glycerine and fatty acids
Figure 1: Hydrolysis of triglycerides into glycerine and fatty acids
Alcoholysis Process
If we replace water in the above reaction scheme (figure 1 above) with a glycol, for instance glycerine, we end up with reactions as per Figure 2 to the left where R¹, R² and R³ represent the carbon chains of the fatty acids in the specific oil used. It will be immediately noticeable that the reaction products are a complex mixture of unreacted reactants (oil and glycerine), monoglycerides (glycerine molecules with a single fatty acid ester linkages) and diglycerides (glycerine molecules with two fatty acid ester linkages). Note that 2 of each monoglyceride and diglyceride are stereoisomers of each other such that there are two primary ester monoglycerides and diglycerides and one secondary ester group formed.
One does not obtain 'pure monoglyceride' as some suspect. The ratios of products produced is dependent on the glycol used, the reaction temperature, dwell time and catalyst used.
In actual alkyd manufacture, the exact amount of each component is not necessarily of great interest. What is of importance is the amount of oil converted to monoglyceride and diglyceride. If there is insufficient mono and di glycerides formed when the diacid is added in the second stage of the reaction, it is more than likely that the diacid will react with any excessive amounts of glycol added in the first stage resulting in gelation of the reactants (gelation occurs when a polymer is so large it becomes insoluble in the mix).
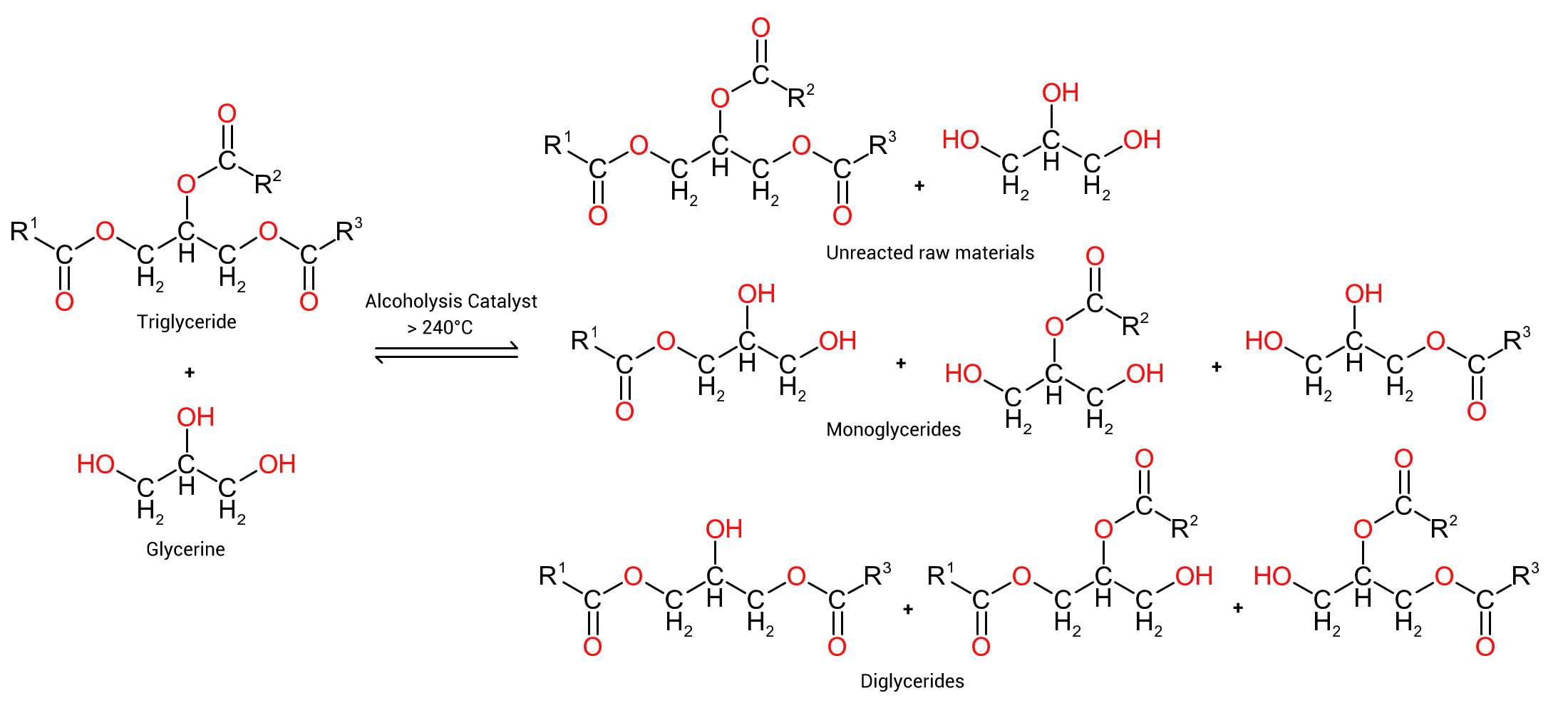
Table 1 is an example of the percentages of mono and diglycerides produced in an alcoholysis reaction with calcium hydroxide and litharge catalysts(Shibayama, K Mitsubishi Denki Laboratory report 1, 1960). Unfortunately neither the reaction conditions nor the oil and glycol used, are available at this time.
| Catalyst | Monoglyceride | Diglyceride | Triglyceride | Glycerine |
|---|---|---|---|---|
| Ca(OH)₂ | 47.4 | 26.1 | 9.7 | 16.8 |
| PbO | 27.1 | 27.1 | 25.2 | 20.6 |
Alcoholysis Catalysts
The catalysts used in alcoholysis are inorganic metal compounds or metal salts of carboxylic acids. The initial choice of a metal catalyst is based on the glycol used in the alcoholysis stage. Where the glycol is pentaerythritol, a solid material that only melts at 276°C, which is normally in excess of the alcoholysis temperatures used, and is almost insoluble in the natural oils used in alcoholysis, a metallic catalyst that is soluble in the oil being used is indicated. Conversely, if a liquid glycol is used in the alcoholysis stage, then a metallic catalyst that is soluble in the glycol is indicated.
| Anion | Metal Compound | Phase Solubility | Comments |
|---|---|---|---|
| Oxides | CaO, PbO, ZnO | Both | Lead oxide (Litharge) was the preferred alcoholysis catalyst for many years but not often used these days due to its toxicity and ecological problems. The effectiveness of the oxides might be dependent upon contaminants in the oils or glycols used, for instance free fatty acid and water contents respectively. |
| Octoates and other carboxylates | Ca, Li, Na, Pb | Oil | General purpose alcoholysis. Some calcium carboxylic acid salts are better than others. The lithium carboxylates are expensive and may also suffer from residual product haze. Lead, once again, is problematic due to toxicity and environmental problems. |
| Hydroxides | Ca(OH)₂, LiOH, NaOH | Glycol | Good general purpose alcoholysis catalysts. Sodium hydroxide is excellent when used with glycerine but can cause darkening of the alcoholysis mix. As noted above, lLithium is now very expensive and can cause a problematic haze in the final product. |
Alcoholysis Temperature
The temperature requirements for alcoholysis can be anything between 240 and 260°C or higher. The alcoholysis will, of course, take longer depending upon the temperature used. What temperatures are used depends mostly on the heating equipment installed as many cannot attain more than 250°C due to heating constraints.
The other dependent is the particular oil/glycol combination used and the associated catalyst. In general, holding at a temperature of 245°C for 45 to 60 minutes should be appropriate. Longer alcoholysis times are indicative of too little catalyst or 'catalyst poisoning', which can occur if the reactor is not properly cleaned after a subsequent batch.
Testing for alcoholysis
There are two methods of testing an alcoholysis mix for completion (or more precisely, confirmation that the alcoholysis mixture is ready to accept the second stage acid addition):
Note that in the rough procedural guide that follows, one is handling samples at very high temperatures and suitable personal protective equipment (PPE) must be used to avoid burns and inhalation of vapours. Additionally, sample handling and testing should be carried out in a fume cupboard, wherever possible.
Standard method
The standard test is the most reliable as it simulates the actual compatibility between the alcoholysis mix, and the diacid to be added. The methodology is as follows:
Assuming that, in a recipe, the alcoholysis mix quantity (i.e. oil and glycol) is 3,000 Kg and 1,000 Kg of phthalic anhydride (i.e. the ratio of alcoholysis mix to phthalic anhydride is 3:1 by mass) is to be added in the second stage, after alcoholysis :
- Using the ratio of 3:1, add 3 parts of the alcoholysis mix (e.g. 300 grams) to a suitably sized glass beaker (e.g. 600ml).
- Add the 1 part (100 grams) of phthalic anhydride to the beaker, place on a suitable hot plate, and begin carefully stirring with a 200°C (or higher) thermometer.
- If the mixture becomes clear before the temperature reaches 155°C then the alcoholysis mixture is ready for the second stage phthalic anhydride addition.
- Note that the alcoholysis/diacid mixture is expected to darken quite substantially as it is heated. This is simply a reaction between the prepared mixture and oxygen in the air above the beaker.
Alternative method
The secondary test for alcoholysis is less precise and more difficult to perform, especially when dealing with oil/pentaerythritol alcoholysis, but is a more rapid indication of alcoholysis completion. The methodology is as follows:
- For an alcoholysis where glycerine is used:
- Using a 10ml measuring cylinder, add 3 parts of AR methanol followed by 1 parts of hot alcoholysis mixture.
- Placing one's thumb over the measuring cylinder opening, shake vigorously for approximately 3 seconds.
- Allow the mixture to cool.
- If the mixture remains clear after shaking, with no signs of separation, the alcoholysis is completed.
- For an alcoholysis where pentaerythritol is used:
- Using a 10ml measuring cylinder, add 3 parts of AR methanol followed by 1 part of alcoholysis mixture straight from the reaction vessel.
- Placing one's thumb over the measuring cylinder opening, shake vigorously for approximately 2 seconds. The mixture needs to be boiling hot (the boiling point of methanol is 65°C and the methanol/alcoholysis temperature will be similar), otherwise the pentaerythritol will rapidly precipitate out masking the alcoholysis test. For this reason, the test is normally carried out right next to the reaction vessel sampling point.
- If the mixture remains clear immediately after shaking, with no signs of separation or precipitation, the alcoholysis is completed.
- This test requires a degree of skill and knowledge when testing an alcoholysis containing pentaerythritol. As such, when using pentaerythritol, the standard test method is preferable.
It cannot be stressed enough that the correct implementation of the alcoholysis test is very important. If it is incorrectly performed it might result in gelation in the second stage, after the diacid and other ingredients are added. Gelation may be rapid, perhaps in 30 minutes or so, post addition.