| Name | Short Form | Structure | Molecular Mass (g.mole⁻¹) | Carbon Atoms | Acid Functionality | Density (20°C) | Flash Point (°C) | Melting Point (°C) | Boiling Point (°C) |
| ALIPHATIC SATURATED ACIDS |
|---|
| Adipic Acid | AA | 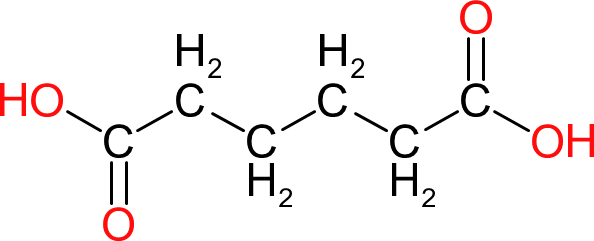 | 146 | 6 | 2 | 1.36 | 196 | 152 | 338 |
| Generally used to increase the flexibility and elongation at break of saturated and unsaturated polyesters but has also found some limited use in alkyds. Adds flexibility without reducing hydrolytic stability to a great degree. |
| Azelaic Acid | | 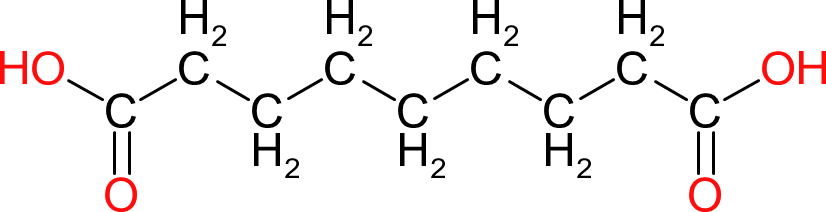 | 188 | 9 | 2 | 1.44 | 210 | 106 | 286 |
| Similar to adipic acid but the longer chain length adds even greater flexibility to the polymer. The ester groups formed also have very good resistance to hydrolysis. Expensive. |
| Sebacic Acid | | 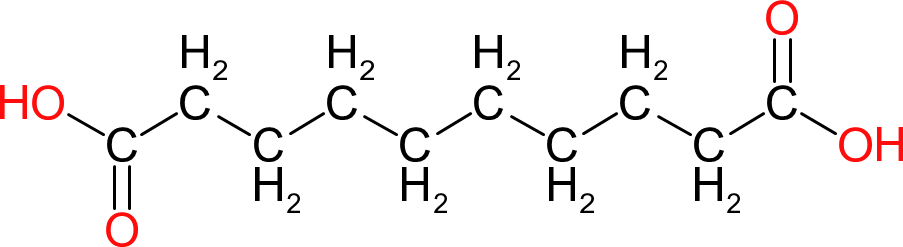 | 202 | 10 | 2 | 1.21 | 220 | 133 | 294 (100mm hg) |
| Similar to Azelaic acid but the even longer chain length provides even greater flexibility. The ester groups formed also has very good resistance to hydrolysis. Expensive. |
| ALIPHATIC UNSATURATED ACIDS |
|---|
| Fumaric Acid | FA | 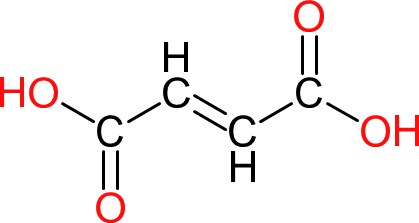 | 116 | 4 | 2 | 1.63 | 230 | 300 | Sublimes at ≥ 200 |
| Used as a source of unsaturation in unsaturated polyesters. More hydrolytically and chemically inert than it's cis analogue (maleic acid). |
| Maleic Anhydride | MA | 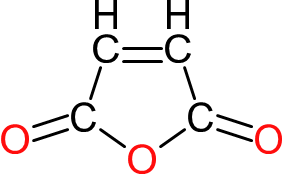 | 100 | 9 | 2 | 1.48 | 157 | 119 | Sublimes |
| Used as a source of unsaturation in unsaturated polyesters. Favoured over maleic acid because of the ease of reacting the anhydride. The 'anhydride ring opening', with bases, produces no water. Once the anhydride ring opens, some of the maleic half-ester formed converts from maleic to the cis isomer, fumaric acid, during processing |
| Itaconic Acid | | 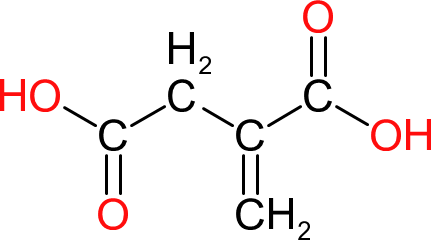 | 130 | 5 | 2 | 1.57 | 199 | 167 | 268 |
| Can be used as a source of unsaturation in polyesters but is rarely done because of the relatively high price. However, being a 'plant sourced' raw material it is a more environmentally friendly option to maleic anhydride. |
| AROMATIC UNSATURATED ACIDS |
|---|
| Benzoic Acid | BA | 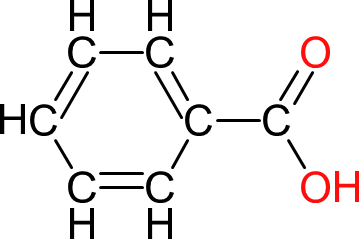 | 122 | 7 | 1 | 1.48 | 121 | 123 | 249 |
| Used as a 'chain stopper, to reduce the molecular mass of the polymer. The aromatic ring also adds to the hardness of the polymer. |
| Phthalic Anhydride | PA | 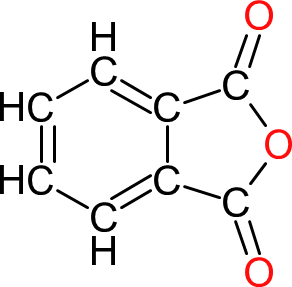 | 148 | 8 | 2 | 1.53 | 152 | 132 | Sublimes |
| The 'standard' diacid used in the majority of alkyds and other polyesters as it is the cheapest diacid available. Like maleic anhydride, the initial 'ring opening' stage, where the anhydride group reacts with a base, produces no water. However, the ester groups formed with phthalic anhydride and bases do not have particularly good hydrolytic stability. |
| Isophthalic Acid | IPA/PIA | 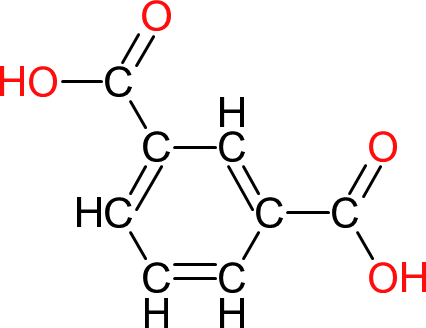 | 166 | 8 | 2 | 1.54 | -- | 345 | Sublimes |
| One of the isomers of phthalic anhydride (acid). As it has no anhydride group, reactions with isophthalic are slower than phthalic anhydride and produce more water. However Isophthalic acid greater hydrolytic stability to the ester groups formed. |
| Terephthalic Acid | TA | 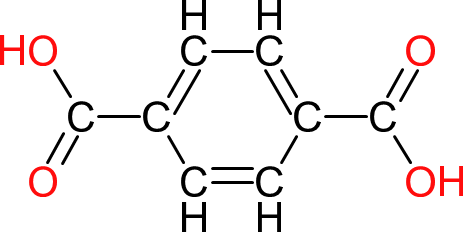 | 166 | 8 | 2 | 1.58 | 260 | 402 | Sublimes |
| The second of the isomeric analogues of phthalic anhydride (acid). Like isophthalic acid, reactions with terephthalic acid are slower and produce more water. The carboxyl groups on terephthalic acid are also less reactive which results in even slower formation of ester groups. A further downside of terephthalic acid is that polymer chains tend to be linear and can form crystals that can precipitate out of the final product. |
| Trimellitic anhydride | TMA | 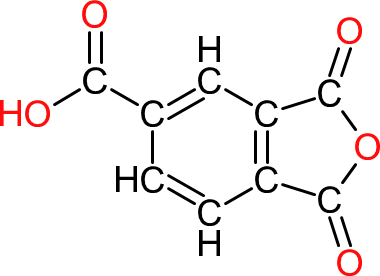 | 192 | 9 | 3 | 1.53 | 227 | 168 | 390 |
| TMA is used to increase the crosslinking of alkyds, saturated and unsaturated polyesters through its additional acid functionality . It is also used to formulate water soluble or water emulsifiable alkyds. |