Modified Alkyd Resins
General
The The standard alkyd resins may be modified, by either condensation reactions or addition reactions, to provide surface coatings resins with enhanced properties more suited to specialised applications. Alkyds thus modified may be either the air-drying types, those that require a catalyst or other reactive polymer, or those that require subsequent drying in a heated oven, to produce the final coating.
Chain Stopped Alkyds
I debated whether to put chain stopped alkyds in the section on alkyds, as the chemistry is very similar, or modified alkyds, but finally chose the later. Medium and short oil alkyds can be modified to provide somewhat similar resins but with their properties chemically altered for specific applications. The alteration to the resin is, as the name implies, by a chemical process known as 'chain stopping'. The synthetic chemicals used in standard alkyds (long, medium and short) are typically those that are 'multifunctional' in that each molecule that is added to form the polymer contains more than one reactive group. In this way the acids and bases form long polymer chains such as -A-B-A-B-A-B-A-B-, where A is a difunctional acid and B a difunctional base. If the reactants were added in equal ('stoichiometric' - i.e. a 1:1 ratio) quantities then the polymer would end up being infinitely large and not soluble in any solvent, something called a 'gel' in resin terms. Thus it is necessary to ensure that more of one component is added such that the reaction can go so far, and no further. In the majority of alkyds the base (hydroxy containing monomer) is added in excess such that the polymer resembles something akin to -B-A-B-A-B-A-B- with little to no additional acid available for further reaction. As the resin polymer is terminated by a base these resins are called 'hydroxy terminated' resins. Another way of terminating the reaction is by adding a monoacid monomer, a molecule with only a single acid group. Benzoic acid is one frequently used monoacid in these reactions. If we represent benzoic acid by 'BA' then the polymer chain might look something like -B-A-B-A-B-A-B-BA, where the benzoic acid prevents the chain from extending on the right side whilst the left side is the normal hydroxy termination.
The chain stopped alkyds are produced in an identical manor to the standard alkyds except that the 'chain stopper' is added during the condensation reaction, along with the other ingredients. The addition of monoacids, like benzoic acid, reduces the viscosity of the final resin but also increases the hardness of the dried resin (coating) film. The lower viscosity of the chain stopped alkyds provides coatings with enhanced coating thicknesses and roughly half the drying time of the equivalent alkyd of the same oil length.
Chain stopped medium oil alkyds are normally supplied in white spirits solvent and used for brushing or spray primers and enamels for the coating of mild steel, particularly machinery and agricultural equipment. Oxidative drying chain stopped short oil alkyds are used in fast drying industrial coatings applied by spray or mechanical roller in the same applications as those of the styrenated alkyds below. They do, however have superior UV, yellowing and cracking resistance, improved adhesion to mild steel, as well as shorter periods before they can be overcoated, than do the styrenated alkyds.
Styrenated Alkyds
Styrenated alkyds are typically based on similar chemistry to the long and medium oil alkyds that are based on oxidative drying oils and fatty acids. These resins are hybrid in nature and are co-reacted with styrene monomer, via an addition reaction, to produce the final resin. Although the presumption is that the final product is composed of entirely of an alkyd backbone to which styrene is attached, the final products are generally a mixture of unreacted alkyd, styrenated alkyd and polystyrene chains.
The solvent used for styrenated alkyds is invariably a fast evaporating type, mainly xylene, although other aromatic and/or oxygenated solvents are also possibilities.
Coatings based on styrenated alkyds are utilised coatings normally applied by spray, or dip, methods where the fast surface drying chacteritics allow rapid handling of the coated item after the coating has been applied. Touch dry times are normally of the order of 5 to 10 minutes, but where coatings are designed to produce 'hammertone' finishes, a slower drying styrenated alkyd (approximately 15 to 25 minutes surface dry) is used to allow the 'hammer' effect to develop before the surface dries.
Styrenated alkyds generally contain from 25% to 40% of styrene by mass, the original quantity of the oil/fatty acid in the hybrid product is lowered by the same margin. The lower quantity of drying oil in the final product thus reduces the number of active sites for oxidative cross-linking and the ultimate hardness of the coating takes a considerably longer period to achieve, even though the product might be handleable in a matter of minutes. One of the downsides of this is that it makes a freshly painted surface difficult to 'touch-up' unless done within approximately 4 hours of the original coating being applied. After ±4 hours, the oxidative cross-linking of the coating will have progressed to such an extent that the solvents used in the coating will often soften the previous applied coating but not dissolve it. When this happens, the softened film has a tendency to 'wrinkle' (also known as 'alligatoring') and possibly crack.
The other 'downsides' of styrenated alkyds are that, because the films are very hard, they can chip fairly easily. Coatings based on styrenated alkyds also do not have very good exterior durability nor do they have very good yellowing resistance when continuously subjected to dark conditions.
Styrenated alkyd coatings are used as a temporary, 'transport', primer for mild steel, metal office furniture finishes, bicycle frames and numerous other areas where a quick drying coating is required to ensure rapid handling of the final coated product, as found on assembly lines.
Vinyl Toluene (VT) modified Alkyds
Much the same can be said about VT alkyds that has already been said about the styrenated alkyds, they are produced in a similar way and they are used in similar applications. The difference between styrenated alkyds and VT alkyds is that the modification of the alkyd with vinyl toluene produces a polymer with improved solubility in the lower solvency, slower evaporating, solvents, for example mineral/white spirits. The use of a slower evaporating solvent system means that the coatings are slow enough drying that they can used with hand brushes or rollers, and thus suitable for hand painting mild steel items, such as industrial or agricultural machinery.
Acrylated Alkyds
In a similar manner to both the styrenated and VT alkyds, long and medium oil alkyds are modified with acrylic monomers to produce hybrid acrylated alkyds by an addition reaction. Once again, much of which has been said about the styrenated and VT alkyds also pertains to the acrylated alkyds. Where the acrylated alkyds differ, however, is that the use of an acrylic monomer, such as butyl acylate, methyl methacrylate etc, produces a hybrid polymer that provides coatings with improved gloss, colour, colour retention and exterior durability.
The cost of the acrylic and methacrylic monomers is much higher than that of styrene and thus the final acrylated alkyd is of higher cost. Because of the cost differential, the acrylated alkyds tend to be used in more demanding applications where coatings based on other resins are not suitable. This may be in either can or automotive refinish coatings. The applications where acrylated alkyd coatings are used dictates the formulation of the coating. For example, can coatings are normally applied by roller and subjected to heat treatment to fully harden the finish whereas acrylated alkyds used in automotive refinish, are mixed with a suitable reactive polyisocyanate, immediately prior to use, that will cross-link with the alkyd component of the coating at room temperature.
Urethanated Alkyds
Appropriate long oil alkyds may be further modified with isocyanates, via an addition reaction, to produce urethane linkages and thus 'urethane alkyds'. The long oil alkyds used for this process are formulated in the same way that the normal long oil alkyds are, although with a lower molecular mass, and with more available free hydroxyl groups, than is normally the case. The isocyanates used in this instance are those molecules that contain dual isocyanate groups, otherwise known as 'diisocyanates', whilst the rest of the diisocyanate molecule may be either aliphatic or aromatic in nature. The reaction of the diisocyanate with the available alkyd hydroxyl groups produces urethane linkages that 'bridge' the alkyd polymer chains together, thus effectively doubling the size of the polymer molecules, whilst maintaining polymer solubility in the customary white/mineral spirits solvent. The reasons behind producing urethane alkyds is that the addition of isocyanate provides coating films with greater toughness and chemical resistance. The enhanced properties of the urethane alkyds make them more suitable for use where a higher degree of abrasion, water, or chemical resistance is required. Typical uses of coatings based on urethane alkyds are wooden furniture varnishes applied by brush (particularly table tops, where the water and heat resistance prevents water marks), alkali resistant plaster primers and concrete floor coatings, anti-corrosion primers, and any wooden items that require a more durable coating, such as skirting boards and doors.
As previously mentioned, the diisocyanate used in the manufacture of a urethane alkyd may have either an aromatic or aliphatic backbones. Urethane alkyds produced with aromatic diisocyanates are not suitable for exterior applications, whilst those aliphatic based diisocyanates are preferred for coatings subjected to external conditions.
Chemistry
Chain Stopped Alkyds
Chain stopped alkyds follow the same formulating and processing principles as do the standard alkyd resins and are simply alkyds that have an additional component, either a monoacid or monobasic component (ie. those monomers with either a single acid (carboxyl) group or a single alcohol (hydroxy) group). The choice of which monomer to use depends on what one is hoping to achieve. For the standard hydroxyl terminated alkyds, a monoacid is used, for those alkyds designed to be acid terminated, then a monobasic monomer is used. The choice of monoacid/monobase is further limited to those acids/bases that have relatively high boiling points and/or azeotrope temperatures with water such that they do not condense off with the water or reaction.
The reasons for adding a monoacid 'chain stopper' to an alkyd is either:
- To impart additional hardness to the resulting coating film Or
- To reduce the alkyd molecular mass
The reasons for adding a monobasic 'chain stopper' to an alkyd is solely to reduce the molecular mass of the alkyd.
The addition of these monomers can either be achieved by:
- Adding, along with the other raw materials, at the beginning of the process (if a single stage reaction) or after the alcoholysis reaction (in a two stage processes) Or
- At some later stage in the process, although there is normally little to gain from using this approach
The monomers generally used as chain stoppers are:
- Monoacids: Benzoic Acid, t-Butyl Benzoic Acid
- Monohydric Alcohols: High boiling point alcohols
Quantities of chain stopper used are normally in the vicinity of 5% to 10% by weight of raw materials charged. Lower quantities will have very little effect on the film hardness whilst higher quantities will result in alkyds of too low molecular mass.
Long oil alkyds are rarely chain stopped, especially with mono acidic monomers, as doing so will reduce the already low hydroxyl content of the completed alkyd, which not only affects their pigment dispersion properties but also their water tolerance (if of importance). Long oil alkyds are traditionally used because of their flexibility and elongation. Adding chain stoppers will reduce the resin elongation which will also reduce the durability and weathering of the coating film
The alkyds that are usually subjected to chain stopping are the medium and long oil alkyds. Chain stopping these alkyds reduces the alkyd drying times (more specifically the 'touch dry' time) and imparts a greater hardness to the coating film. Touch dry times for medium oil alkyds can be reduced from circa 1 ½ to 2 hours to around 30 to 40 minutes, whilst short oil alkyds can exhibit reduced touch dry times from 1 hour to 10 to 15 minutes.
Urethanated Alkyds
Alkyds can also be modified with isocyanates (normally diisocyanates) to improve the properties of the base alkyd which is being modified. The addition of diisocyanates improves the following alkyd properties:
- The resultant coating hardness, durability and water resistance
- Reduction of the alkyd viscosity at a similar target molecular mass to a non-modified alkyd
- Adds some degree of adhesion and anti-corrosive properties when used in primers
Whereas with a chain stopped alkyd it is possible to add the chain stopper monomer to an existing alkyd formulation without any, or little modification to the base resin, the alkyd base resin used in the manufacture of a urethane alkyd has to be specifically designed to accommodate the addition of a diisocyanate.
There are two types of diisocyanate used:
- Aromatic Diisocyanates: such as 80/20 Toluene Diisocyanate (TDI). Produces hard durable films but not recommended in resins for exterior applications.
- Aliphatic Diisocyanates: such as hexamethylene diisocyanate (HDI). Do not darken as much as aromatic diisocyanates and have superior exterior durability. More expensive than the aromatic diisocyanates though.
The reaction sequence to the right is based on the reaction between an alkyd intermediate, based on the four hydroxy functional pentaerythritol, with associated diacid and fatty acid components (R¹ and R²), and 2,4 toluene diisocyanate. The reaction requires a temperature of between 70°C to 80°C and results in the formation of two urethane groups linking the two alkyd molecules together.
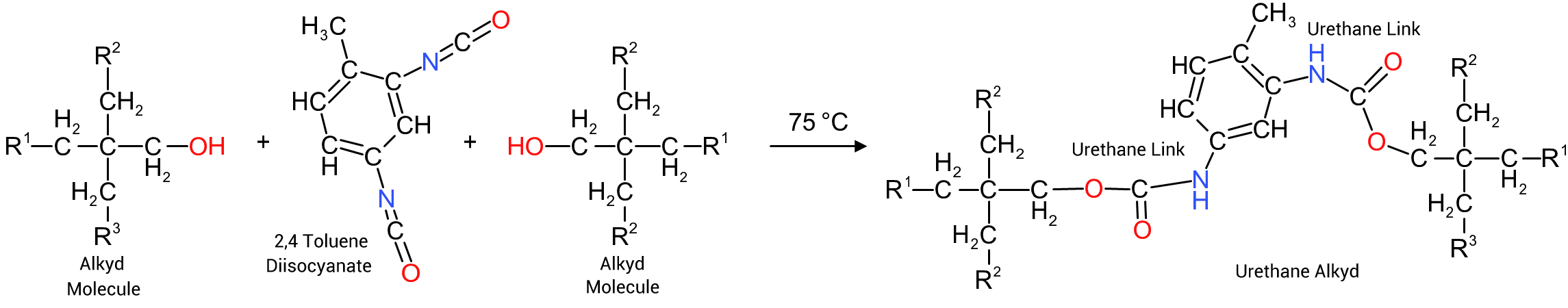
The normal procedure to manufacture a urethane alkyd is:
- Manufacture the base resin alkyd polymer
- Dissolve the base resin in a suitable solvent (the solvent must contain no free hydroxy groups otherwise the diisocyanate will almost certainly preferentially react with the solvent hydroxy group and not the alkyd hydroxyls) at a specified solids content.
- Heat the resin solution up to a specified temperature.
- Slowly add the required quantity of diisocyanate to the resin solution ensuring the solution temperature does not excessively increase (the reaction is highly exothermic) above a pre-determined maximum temperature
- Allow the reaction to go to completion by measuring the free Isocyanate value (should be less than 1 mg KOH/g on solids content)
- Reduce the completed urethane alkyd to the required viscosity specification.
Notes:
- The isocyanates are extremely toxic and adequate methodology is required in its handling, both in terms of the equipment used and full PPE for those involved in the urethane handling and manufacture.
- It is imperative that there are excess hydroxyl groups in the base resin to react with the added quantity of diisocyanate. An excess amount of diisocyanate will result in free isocyanate mixed in the finished resin, which will not only cause skinning of the resin but also renders the resin toxic! If the required viscosity is not met with the prescribed amount of isocyanate, do not be tempted to see if the viscosity will go any higher by adding more diisocyanate!
Acrylated Alkyds
Information on the Acrylated, styrenated and VT alkyds will follow after some preliminary information on additional reactions